Halozyme Therapeutics (NASDAQ: HALO) is a biotechnology company focused on developing and commercializing novel oncology therapies that target the tumor microenvironment. Halozyme’s lead proprietary program, investigational drug PEGPH20, applies a unique approach to targeting solid tumors, allowing increased access of co-administered cancer drug therapies to the tumor in animal models.
PEGPH20 is currently in development for metastatic pancreas cancer, non-small cell lung cancer, gastric cancer, metastatic breast cancer and has potential across additional cancers in combination with different types of cancer therapies.
During June 2017, the company presented encouraging results from a Phase 2 randomized, multi-center clinical trial in pancreas cancer patients. The study met its primary endpoints and the key secondary endpoint of progression-free survival (PFS) in patients with high levels of a biomarker, hyaluronan (HA-High). Halozyme is currently conducting a global Phase 3 clinical trial, HALO-301, in a similar population of HA-High stage IV pancreas cancer patients.
As per management, HALO is driven in its study of PEGPH20 by the goal of providing new options to patients affected by some of the hardest to treat cancers. The HALO-202 results in HA-High pancreas cancer patients provide encouraging insights into a potentially new treatment option and support its ongoing study of PEGPH20 in the global Phase 3 HALO-301 study.
Pancreas cancer is the third-leading cause of cancer related death in the United States, and more than 65,000 people in the U.S. and top five European countries are diagnosed annually with advanced cases of the disease.
The company also announced that an Oncologic Drug Advisory Committee of the U.S. Food and Drug Administration voted 11 to 0 that the benefit/risk of rituximab/hyaluronidase for subcutaneous (under the skin) injection was favorable for patients in the proposed indications of follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia. The FDA action date is tentatively June 26, 2017. This is one of the major potential near term catalyst for the company.
As per the company, subcutaneous rituximab can be administered in five to seven minutes compared to an hour and a half or more for intravenous Rituxan. The significant reduction in administration time could especially benefit people with blood cancer who may receive years of treatment, and therefore the committee unanimously supported this new co-formulation.
Rituximab co-formulated with Halozyme’s recombinant human hyaluronidase was approved in Europe in 2014 and is currently marketed as the subcutaneous (SC) formulation of MabThera® (rituximab) in approximately 50 countries worldwide.
The European approved version of the similar drug, Mabthera, resulted into $7.32 billion dollars in revenue during 2016. The treatment for two extremely common cancers which patients are treated for years has extreme potential. If we factor in that the IV treatment version of the composition takes nearly 20x as long as this subcutaneous version. This creates an attractive value proposition for the company and its investors.
The Company announced its quarterly earnings data on Monday, May 15th. Revenue for the first quarter was $29.6 million compared to $42.5 million for the first quarter of 2016. Net loss for the first quarter was $32.9 million, or $0.26 per share, compared to net loss in the first quarter of 2016 of $19.8 million, or $0.16 per share.
Notwithstanding the muted results, core fundamentals (over the longer term) of the company continue to remain promising & the management is pleased with the Company’s momentum as it makes progress towards launching its promising pipeline.
With the recent developments, analyst have revised their outlook on the stocks:
Firm: Rating: Price: Date:
Deutsche Bank AG BUY $16.00 Mar’30th
Zacks HOLD $16.00 May’12th
The stock currently has an average rating of “BUY” and a consensus price target of $16.77. Considering present valuation, HALO is at a favorable risk reward position.
About the Company: Halozyme Therapeutics is a biotechnology company focused on developing and commercializing novel oncology therapies that target the tumor microenvironment. Halozyme’s lead proprietary program, investigational drug PEGPH20, applies a unique approach to targeting solid tumors, allowing increased access of co-administered cancer drug therapies to the tumor in animal models. PEGPH20 is currently in development for metastatic pancreatic cancer, non-small cell lung cancer, gastric cancer, metastatic breast cancer and has potential across additional cancers in combination with different types of cancer therapies. In addition to its proprietary product portfolio, Halozyme has established value-driving partnerships with leading pharmaceutical companies including Roche, Baxalta, Pfizer, Janssen, AbbVie, and Lilly for its ENHANZE® drug delivery technology. Halozyme is headquartered in San Diego.
Major pipeline
Lead Phase 3 Oncology Asset: PEGPH20: Targets and degrades Hyaluronan, a recognized prognostic biomarker, and barrier to effective treatment of pancreas cancer, Positive randomized Phase 2 data with a validated companion diagnostic, Phase 3 trial ongoing in target population, projected potential filing 2018-2020
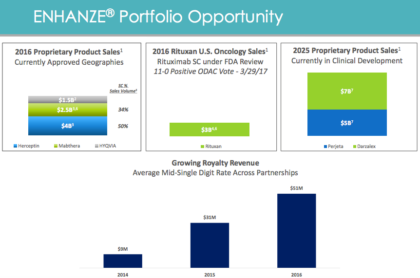
ENHANZE®: Growing royalty revenue with potential for near-term revenue inflecting events
Recent Highlights:
- Enrollment tracking to plan in HALO-301, the company’s Phase 3 study of pancreas cancer patients at over 200 global sites in 22 countries.
- Announcing an Oncologic Drug Advisory Committee of the U.S. Food and Drug Administration voted 11 to 0 that the benefit/risk of rituximab/hyaluronidase for subcutaneous injection was favorable for patients in the proposed indications of follicular lymphoma, diffuse large B-cell lymphoma, and chronic lymphocytic leukemia. The FDA action date is June 26. Analysts estimate rituxumab sales in oncology indications in the United States were approximately $3 billion in 2016.
- Advancing the development of multiple products using Halozyme’s ENHANZE® technology. Roche continues in its ongoing Phase 1 study to examine the combination of Herceptin SC and a subcutaneous formulation of Perjeta using ENHANZE®, including investigation into whether a single injection of the combination can be achieved, potentially providing a significant convenience for patients.
In addition, Janssen has developed a rapid delivery SC formulation of daratumumab using ENHANZE® technology and recently dosed the first patients as part of their ongoing Phase 1 study. Janssen is currently planning to initiate a Phase 3 study using the new formulation later this year.
The collaboration with Lilly using Halozyme’s ENHANZE® technology is progressing across multiple targets with preclinical and clinical studies this year and next.
First Quarter 2017 Financial Results:
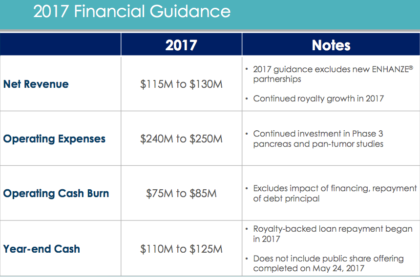
Revenue for the first quarter was $29.6 million compared to $42.5 million for the first quarter of 2016. The year-over-year decrease was driven by $15.5 million received in license and milestone payments from Lilly, AbbVie, and Pfizer in the first quarter of 2016, partially offset by increases in royalties from partner sales of Herceptin® SC, MabThera® SC and HYQVIA®, and research and development reimbursements from ENHANZE® partners.
Revenue for the first quarter included $14 million in royalties, an increase of 23 percent from the prior-year period, $8.2 million in sales of bulk rHuPH20 primarily for use in manufacturing collaboration products and $3.2 million in HYLENEX® recombinant (hyaluronidase human injection) product sales.
Net loss for the first quarter was $32.9 million, or $0.26 per share, compared to net loss in the first quarter of 2016 of $19.8 million, or $0.16 per share. Cash, cash equivalents and marketable securities were $179 million at March 31, 2017, compared to $205 million at December 31, 2016.
Cash, cash equivalents and marketable securities were $179 million at March 31, 2017, compared to $205 million at December 31, 2016.
The amount and timing of cash requirements will depend on the progress and success of the company’s clinical development programs, regulatory and market acceptance, and the resources it devotes to research and commercialization activities.
As per management, current cash, cash equivalents and marketable securities will be sufficient to fund operations for at least the next twelve months. They expect to fund operations going forward with existing cash resources, anticipated revenues from existing collaborations and cash that the company may raise through future transactions.
It may raise cash through any one of the following financing vehicles: (i) the public offering of securities; (ii) new collaborative agreements; (iii) expansions or revisions to existing collaborative relationships; (iv) private financings; (v) other equity or debt financings; and/or (vi) monetizing assets.

Key risk factors and potential stock drivers:
The company is exposed to significant risk of the potential for dilution. The company is going to need incremental capital.
The favorable outcome of upcoming FDA action date on June 26, 2017, could be a near term trigger for the stock.
The company’s ability to ramp-up profitability while sustaining its revenue growth would be one of the key stock driver over the near to medium term.
Stock Chart:
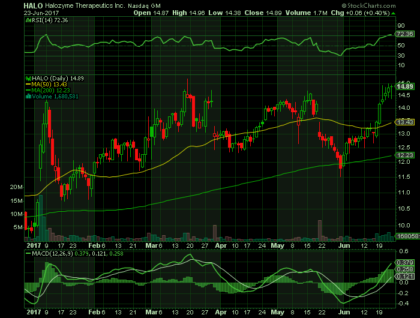
On Friday, June 23rd, 2017, HALO closed at $14.89 (up 0.4%) on volume of 1.7 million shares exchanging hands. Market capitalization is $2.09 billion. The current RSI is 72.36
In the past 52 weeks, shares of HALO have traded as low as $7.70 and as high as $15.20.
At $14.89, shares of HALO are trading above its 50-day moving average (MA) at $13.43 and above its 200-day MA at $12.23
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP – SMS/text alert service for free, simply text the word “Traders” to the phone number “25827” from your cell phone***
Disclaimer and Privacy Policy
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

