Viking Therapeutics, Inc. (NASDAQ: VKTX) is a clinical-stage biopharmaceutical company focused on the development of novel, therapies for metabolic and endocrine disorders. Viking has exclusive worldwide rights to a portfolio of five therapeutic programs in clinical trials or preclinical studies, which are based on small molecules licensed from Ligand Pharmaceuticals Incorporated.
Recent Events
October 24, 2017.Viking announced positive final results from an eight-week study of VK2809 in an in vivo model of non-alcoholic steatohepatitis (NASH). Data from this study demonstrated statistically significant improvements in several key measures relevant to the development and progression of NASH. Additionally, an evaluation of gene expression changes demonstrated statistically significant changes in the expression of multiple genes associated with the development and progression of NASH following eight weeks of treatment with VK2809, as compared to vehicle control.
October 23, 2017. The company announced positive results from a 25-week proof-of-concept study of VK0214 in an in vivo model of X-linked adrenoleukodystrophy (X-ALD). The final study data were presented in a poster presentation at the 87th Annual Meeting of the American Thyroid Association (ATA), held October 18-22, 2017, in Victoria, British Columbia.
The results of this study showed that treatment with VK0214 led to statistically significant reductions in plasma levels of multiple very long chain fatty acids (VLCFAs), including the benchmark highly toxic C26 fatty acid, in treated animals compared with vehicle controls.
October 9, 2017. Viking Therapeutics, Inc. hosted a key opinion leader (KOL) event that featured presentations on VK5211 as well as the hip fracture market, the lack of effective osteoporosis treatments, and regulatory outcomes for therapies targeting sarcopenia and hip fracture.
VK5211 is a third-generation non-steroidal Selective Androgen Receptor Modulator (SARM) that is being developed for maintenance or improvement of lean body mass (LBM), bone mineral density (BMD), and function in patients recovering from non-elective hip fracture surgery.
Products
The VKTX lead clinical program, VK5211, is a novel, orally available, non-steroidal selective androgen receptor modulator, or SARM, in development for the treatment of patients recovering from non-elective hip fracture surgery. VK5211 is designed to produce the therapeutic benefits of testosterone in muscle and bone with improved safety, tolerability and patient acceptance. They have initiated a Phase 2 clinical trial with VK5211 in patients recovering from non-elective hip fracture surgery.
The second pipeline program is focused on the development of novel small molecule agonists of the thyroid beta (TRb) receptor, VK2809 and VK0214. These novel TRb agonists are being developed for hypercholesterolemia and fatty liver disease and X-linked adrenoleukodystrophy, or X-ALD. The company has initiated Phase 2 clinical development of VK 2809 in hypercholesterolemia and fatty liver disease. VK0214 is in preclinical development for X-ALD.
They are also developing VK0612, a first-in-class, orally available drug
About
Viking Therapeutics, Inc. is a clinical-stage biopharmaceutical company focused on the development of novel, first-in-class or best-in-class therapies for metabolic and endocrine disorders. The company’s research and development activities leverage its expertise in metabolism to develop innovative therapeutics designed to improve patients’ lives. Viking has exclusive worldwide rights to a portfolio of five therapeutic programs in clinical trials or preclinical studies, which are based on small molecules licensed from Ligand Pharmaceuticals Incorporated. The company’s clinical programs include VK5211, an orally available, non-steroidal selective androgen receptor modulator, or SARM, in Phase 2 development for the treatment and prevention of lean body mass loss in patients who have undergone hip fracture surgery, VK2809, a small molecule thyroid beta agonist in Phase 2 development for hypercholesterolemia and non-alcoholic fatty liver disease, and VK0612, a first-in-class, orally available drug candidate in Phase 2 development for type 2 diabetes. Viking is also developing novel and selective agonists of the thyroid beta receptor for GSD Ia and X-linked adrenoleukodystrophy, as well as two earlier-stage programs targeting metabolic diseases and anemia.
Analysts
Jul-17-17 Reiterated H.C. Wainwright Buy $5 → $7
Financial Review
Research and development expenses for the three months ended June 30, 2017 were $3.7 million compared to $2.4 million for the same period in 2016. The increase was primarily due to increased activities related to our clinical trials for our VK5211 and VK2809 programs, third party manufacturing of our clinical-stage drug candidates, and preclinical efforts for our VK0214 program.
General and administrative expenses for the three months ended June 30, 2017 were $1.3 million compared to $1.2 million for the same period in 2016. This small increase was primarily due to increases in salaries and benefits and legal expenses, offset by a decrease in non-cash stock compensation expense.
For the three months ended June 30, 2017, Viking reported a net loss of $5.2 million, or $0.21 per share, compared to a net loss of $3.7 million, or $0.22 per share, in the corresponding period in 2016. The increase in net loss for the three months ended June 30, 2017 was primarily due to the increase in research and development expenses noted previously.
Research and development expenses for the six months ended June 30, 2017 were $7.2 million compared to $4.2 million for the same period in 2016. The increase in research and development expenses was primarily related to increases in expenses related to clinical trial activity for our VK5211 and VK2809 programs and preclinical efforts for our VK0214 program, as well as third party manufacturing of our clinical-stage drug candidates.
General and administrative expenses for the six months ended June 30, 2017 were $2.7 million compared to $2.6 million for the same period in 2016. This small increase was primarily due to increases in salaries and benefits, offset by a decrease in non-cash stock compensation expense.
For the six months ended June 30, 2017, Viking reported a net loss of $10.4 million, or $0.45 per share, compared to a net loss of $7.3 million, or $0.56 per share, in the comparable period in 2016. The increase in net loss for the six months ended June 30, 2017 was primarily due to the increase in research and development expenses noted previously.
At June 30, 2017, Viking held cash, cash equivalents and investments totaling $12.1 million. As of July 31, 2017, Viking had 27,697,284 shares of common stock outstanding.
Viking is expected to release its Q3 earnings on or about November 9, 2017.
Stock Influences and Risk Factors
If one or more of their current drug candidates receives regulatory approval or gets commercialized, it would be a significant catalyst;
VKTX is a clinical-stage company, and expects to incur significant operating losses during the next stages of corporate development.
The company is dependent on technologies licensed from Ligand, and if they lose the license, their ability to develop existing and new drug candidates would be harmed.
The biotech space is a high-risk sector due to uncertainties associated with the novel drug development. Any adversities related with the same could upset the stock performance significantly.
Stock Chart
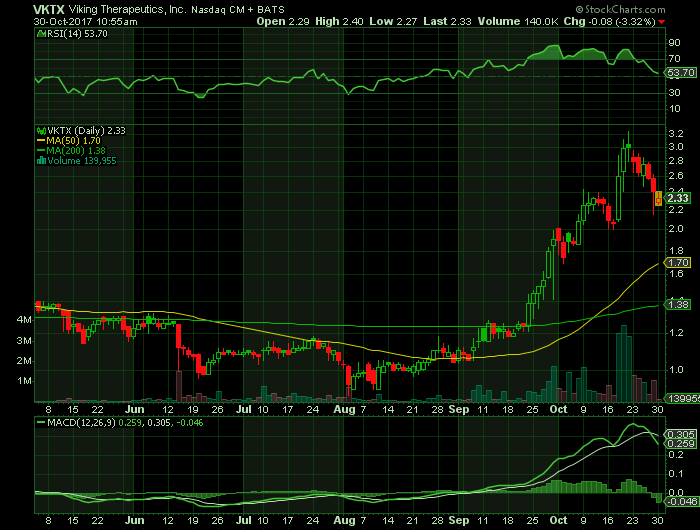
On Monday, October 30, 2017, in intra-day trading, VKTX shares were at $2.33 on traded volume of 140K shares. The current RSI (14) is 53.70.
At $2.33 VKTX shares are trading above their 50-day moving average of $1.70 and their 200-day moving average of $1.38/ share.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

