VIVUS, Inc. (NASDAQ: VVUS), is a biopharmaceutical company, develops and commercializes therapies to address unmet medical needs in the United States and the European Union.
The company has two approved therapies and one product candidate in active clinical development. Qsymia® (phentermine and topiramate extended release) is approved by FDA for chronic weight management and STENDRA® (avanafil) is approved by FDA for erectile dysfunction, or ED, and by the European Commission, or EC, under the trade name SPEDRA in the EU. Tacrolimus is in clinical development for the treatment of Pulmonary Arterial Hypertension, or PAH.
VIVUS has licensing agreements with Mitsibushi Tanabe Pharma Corporation, and the company has entered into several sub-license agreements for most major territories. The sub-licensees consist of the Menarini Group (Europe), Metuchen Pharmaceuticals LLC (United States, Canada, South America, India), and Sanofi (Africa, Middle East, Turkey, Commonwealth of Independent States). Successful distribution of its products is essential to VIVUS’ growth.
VIVUS 2017 Highlights:
September. The company announced that the European Medicines Agency (EMA) has granted Orphan Drug Designation to the Company’s lead clinical candidate tacrolimus, for the treatment of pulmonary arterial hypertension (PAH).
September. VVUS signed an agreement with global, privately owned pharmaceutical company Alvogen giving rights to the latter to market anti-obesity drug Qsymia in Korea for the treatment of chronic weight management or weight-related conditions.
August. VVUS entered into a settlement agreement with Dr. Reddy’s Laboratories, S.A. and Dr. Reddy’s Laboratories, Inc. resolving patent litigation related to Qsymia®. The settlement agreement permits Dr. Reddy’s to begin selling a generic version of Qsymia on June 1, 2025, or earlier under certain circumstances. In the event of a launch earlier than June 1, 2025, VIVUS will receive a royalty on sales of the generic version of Qsymia.
In July, VIVUS announced a settlement agreement with Actavis Laboratories FL (Actavis) resolving patent litigation related to Qsymia®. The litigation resulted from the submission by Actavis of an Abbreviated New Drug Application (ANDA) to the U.S. Food and Drug Administration seeking approval to market generic versions of Qsymia. The settlement agreement permits Actavis to begin selling a generic version of Qsymia on December 1, 2024, or earlier under certain circumstances. In the event of a launch earlier than December 1, 2024, VIVUS will receive a royalty on Actavis’ sales of the generic version of Qsymia.
In May, VIVUS announced the appointment of Thomas B. King to its board of directors.
Products:
QSYMIA® (Kyoo sim ee’ uh) is the only FDA-approved once-daily prescription weight-loss medicine, is approved in the U.S. and is indicated as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of 30 kg/m2 or greater (obese) or 27 kg/m2 or greater (overweight) in the presence of at least one weight-related medical condition such as high blood pressure, type 2 diabetes, or high cholesterol.
Qsymia incorporates a proprietary formulation combining low doses of active ingredients from two previously approved drugs, phentermine and topiramate, and is being commercialized by the Company in the U.S. through a sales force who promote Qsymia to physicians.
Sales of Qsymia have been muted since its launch due to high out-of-pocket cost burden for patients owing to lack of reimbursement for the product. Healthcare providers are often hesitant to treat obesity proactively despite the presence of evidence regarding the cardiometabolic benefits of weight loss among overweight and obese individuals.
A major portion of the company’s revenues are derived from Qsymia.

SPEDRA™. is an oral phosphodiesterase type 5, or PDE5, inhibitor that VIVUS licensed from Mitsubishi Tanabe Pharma Corporation, or MTPC. FDA approved STENDRA in April 2012 for the treatment of ED in the United States. The European Commission (EC) granted the marketing authorization for SPEDRA™ (avanafil) for the treatment of erectile dysfunction (ED) in the European Union (EU). The approval of the marketing authorization application (MAA) by the EC follows the positive recommendation by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in April 2013. SPEDRA is the first new chemical entity (NCE) approved for ED in over a decade. Avanafil is being commercialized in the U.S., EU, and other countries through commercialization collaborators.
Tacrolimus. On September 6, 2017, the company announced that the European Medicines Agency (EMA) has granted Orphan Drug Designation to the Company’s lead clinical candidate tacrolimus, for the treatment of pulmonary arterial hypertension (PAH). Orphan Drug Designation is awarded for the diagnosis, prevention, or treatment of a life-threatening or chronically debilitating condition that is rare (affecting not more than five in 10,000 people in the European Union) or where the medicine is unlikely to generate sufficient profit to justify research and development costs. This designation provides VIVUS with a number of potential incentives in the EU. Tacrolimus was granted Orphan Drug Designation by the U.S. Food & Drug Administration (FDA) for the treatment of PAH in March 2015. VIVUS remains on track to hold a pre-IND meeting with the FDA by the end of 2017.
About VIVUS:
VIVUS, Inc., a biopharmaceutical company, develops and commercializes therapies to address unmet medical needs in the United States and the European Union. The company offers Qsymia for the treatment of obesity as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial body mass index of 30 or greater, or 27 or greater in the presence of at least one weight-related comorbidity, such as hypertension, type 2 diabetes mellitus or high cholesterol; and STENDRA, an oral phosphodiesterase type 5 inhibitor for the treatment of erectile dysfunction. It is also developing Qsymia, which has completed Phase II studies for the treatment of obstructive sleep apnea and diabetes, as well as for other obesity-related diseases, including nonalcoholic steatohepatitis, nonalcoholic fatty liver disease, hyperlipidemia, and hypertension. In addition, the company is developing Tacrolimus, which has completed Phase IIa studies for the treatment of pulmonary arterial hypertension. It has development, license and clinical trial, and commercial supply agreement with Mitsubishi Tanabe Pharma Corporation for the development and commercialization of avanafil, a PDE5 inhibitor compound for the oral and local treatment of male and female sexual dysfunction. The company also has license and commercialization agreements with Berlin-Chemie AG and Auxilium Pharmaceuticals, Inc. to commercialize and promote STENDRA; and with Sanofi Winthrop Industrie to commercialize and promote avanafil. VIVUS, Inc. was founded in 1991 and is headquartered in Campbell, California.
Q2 Financial Review:
Total revenue, net for the second quarters of 2017 and 2016, was $11.2 million and $13.8 million, respectively.
Total cost of goods sold was $3.6 million and $2.6 million in the second quarters of 2017 and 2016, respectively. The increase was primarily a result of higher STENDRA/SPEDRA supply revenue during the quarter.
Research and development expense was $1.0 million and $1.1 million in the second quarters of 2017 and 2016, respectively. Research and development expenses were impacted by a decrease in efforts surrounding our Qsymia regulatory requirements partially offset by development efforts of tacrolimus for the treatment of pulmonary arterial hypertension.
General and administrative expense was $6.2 million and $7.7 million for the second quarters of 2017 and 2016, respectively, while selling and marketing expense for the commercialization of Qsymia totaled $5.4 million and $6.0 million in the second quarters of 2017 and 2016, respectively. The decreases were due to the continued cost control initiative and the result of the realignment of our sales force, and refinement of our marketing and promotional programs.
Net loss for the second quarter of 2017 was $13.4 million, as compared to $11.4 million in the second quarter of 2016. Cash, cash equivalents and available-for-sale securities were $251.5 million at June 30, 2017.
Potential stock catalysts and risk factors:
Expanding the use of Qsymia through targeted patient and physician education;
obtain marketing authorization by the EC for Qsymia™ in the EU through the centralized marketing authorization procedure;
may not be able to successfully develop, launch and commercialize tacrolimus or any other potential future development programs;
collaborators may experience financial, regulatory, or operational difficulties, which may impair their ability to commercialize their drug products;
the company has in-licensed all or a portion of the rights to Qsymia and STENDRA from third parties. If they default on any material obligations under those licenses, they could lose rights to those drugs.
Stock chart:
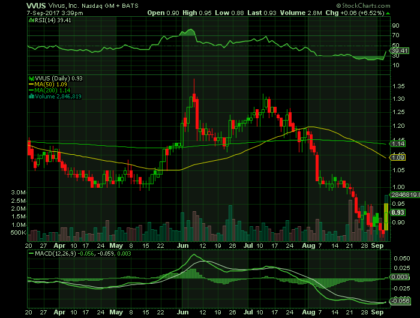
At 3:39 pm on Thursday September 7, 2017, VVUS shares were at $.93 (+6.52%) on volume of 2.8 million shares, sharply higher than the average daily volume of 898 thousand shares. VVUS shares are trading below their 50-day and 200-day moving averages of $1.09 and $1.14 respectively. The RSI (14) is 39.41.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

