| Good day everyone,
Citius Pharmaceuticals, Inc. (NASDAQ: CTXR), is a late-stage biopharmaceutical company focused on oncology, anti-infective products in adjunct cancer care, unique prescription products, and stem cell therapies.
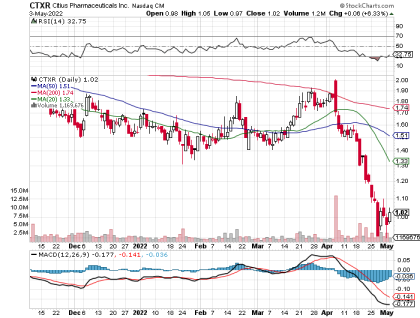
Current price $.9732/share (as of market close 5-5-22)
The NASDAQ had an abysmal day yesterday shedding 647 points or 4.99% of its value. CTXR shares followed the index in lockstep. There was an above average trading volume of 2.3M shares.
CTXR shares look oversold to me. While the NASDAQ index has dragged the value of many companies down, I think the value at CTXR could rise above the fray and here is why:
Book value (mrq) $.80/share
Cash (mrq) $.45/share
Analysts rating “buy”
Analysts target $5.00/share
52-week high $4.56/share
Price on April 6th $2.01/share
With two products in later stage Phase III trials and one in a Phase II clinical trial, CTXR is more developmentally advanced than it ever has been. I want to note that none of the CTXR clinical trials have encountered adverse events (beyond a slowdown due to the pandemic). When Mino-Lok gets to the market, it will be the only FDA approved drug therapy for infected Central Venous Catheters with a global market estimated at $1.84B.
Let’s review the upcoming potential catalysts for CTXR:
Complete enrollment in Mino-Lok Phase 3 trial (end of 2022)
Report Topline results of I/ONTAK Phase 3 trial (April 2022)
Submit I/ONTAK BLA application (2H 2022)
Initiated Halo-Lido Phase 2b trial (April 2022)
Complete enrollment in Halo-Lido Phase 2b trial (end of 2022)
CTXR is developing Mino-Lok under license from the MD Anderson Cancer Center at the University of Texas in Houston. U.S. News & World Report has listed MDACC as one of the nation’s top two hospitals for cancer care every year since the survey began in 1990. CTXR is also in a development partnership with MDACC for their pre-clinical Mino-Wrap product.
In the Phase 2b trial, Mino-Lok® demonstrated a 100% efficacy rate in salvaging colonized CVCs.

Infected double lumen catheter showing biofilm in both lumens.
Leonard Mazur, Chairman and CEO of Citius has Said, “Our Mino-Lok program continues to advance in accordance with the recommendations of the independent data monitoring committee, which advised us to continue with the trial as planned, following each of its three data reviews. We remain encouraged by the positive signal conveyed by the DMC guidance to proceed. Coupled with the recent ramp up in patient recruitment following an easing of COVID-related hospital restrictions, we believe our efforts to increase engagement with existing trial sites and to onboard additional sites will continue to drive trial enrollment and enable us to achieve the necessary trial events to support statistically significant results.”
Mino-Lok may become a blockbuster product for the company with the US market alone estimated at $750M, but the savings to the US healthcare system, currently paying to remove and replace infected CVCs could be in the billions of dollars.
I/ONTAK to treat persistent or recurrent cutaneous T-cell lymphoma is also in a Phase III clinical trial. This drug was previously approved by the FDA in the US as ONTAK but withdrawn from the market several years ago due to manufacturing issues. New manufacturing technology has resolved those issues and CTXR has obtained a license for the product covering most of the world.
I/ONTAK has been approved in Japan (CTXR is not licensed there) and is currently in the market there. I/ONTAK has Orphan Drug Designation from the FDA and the company recently released positive top line data from the current study.
Halo Lido would be the first FDA approved prescription drug to treat hemorrhoids with a global market estimate of $2B. It would also be the only drug from CTXR to go direct to consumers by prescription. Halo Lido is a proprietary CTXR formulation and not being developed under license.
CTXR products in pre-clinical development:
CITI-101 (Mino-Wrap), is a liquefying gel-based wrap containing minocycline and rifampin designed to provide inflammatory tissue protection and prevent infection and biofilm formation in tissue expanders and breast implants post-mastectomy.
Through its subsidiary, NoveCite, Inc., Citius is developing a novel proprietary mesenchymal stem cell treatment derived from induced pluripotent stem cells (iPSCs) for acute respiratory conditions (ARDS). ARDS is the condition thar requires Covid-19 patients to go on a ventilator.
When I consider the huge cash position ($65M-mrq) at CTXR, and two products, (Mino-Lok and I/ONTAK) looking at the FDA finish line, I can only conclude that with a current market cap of only $142M this company may be oversold.
The Traders News Group
original report below
Citius Pharma (CTXR) is a Biopharma Company That Looks Way Oversold with Catalysts Lining Up in Clinical Trials
Good day everyone,
Citius Pharmaceuticals, Inc. (NASDAQ: CTXR), is a late-stage biopharmaceutical company focused on oncology, anti-infective products in adjunct cancer care, unique prescription products, and stem cell therapies.
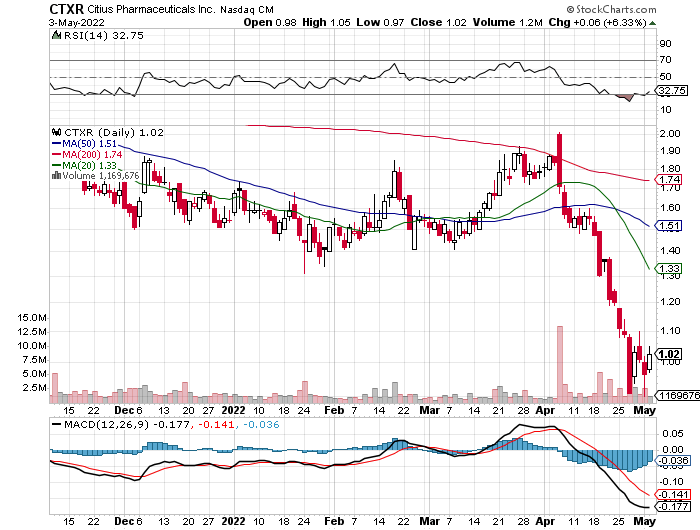
Current price $1.00/share (as of 10:00 a.m. EDT 5-5-22)
After closing the Wednesday session at $1.03/share, CTXR shares are feeling the pressure of an early downslide in the NASDAQ index. Trading volume in the first 30 minutes of today’s session was 537K.
New and upcoming catalysts for CTXR:
Reported Topline results of I/ONTAK Phase 3 trial (April 2022)
Submit I/ONTAK BLA application (2H 2022)
Initiated Halo-Lido Phase 2b trial (April 2022)
Complete enrollment in Halo-Lido Phase 2b trial (end of 2022)
Complete enrollment in Mino-Lok Phase 3 trial (end of 2022)
Mino-Lok would be the only FDA approved drug to treat infected Central Venous Catheters with a global market of estimate of $1.84B
Halo Lido would be the first FDA approved prescription drug to treat hemorrhoids with a market estimate of $2B.
I/ONTAK to treat persistent or recurrent cutaneous T-cell lymphoma. Previously approved in the US as ONTAK but withdrawn due to manufacturing issues. Issues resolved and currently in a phase III trial with a market value yet to be determined.
CTXR has a market cap of only $147M (and they have 65M in cash-mrq) and the company looks to me like a bargain given the late-stage drugs they have in clinical trials and the market potential for each.
Stay tuned for more information on CTXR.
The Traders News Group
original report below
Mino-Lok Could Be a Blockbuster for Citius Pharma (CTXR)
MIno-Lok a potential blockbuster product has been in phase 3 trials since 2018!
The market potential for Mino-Lok is estimated at over $750 million per year in the U.S. alone and is projected to reach $1.84 billion globally by 2028.
Good day everyone,
Current price $1.00/share (at market close 5-4-22)
Currently, CTXR is developing several product candidates but today I want to focus on one, Mino-Lok. While all the therapies in development at CTXR are exciting, I believe Mino-Lok may be the first blockbuster product for the company. Mino-Lok is the product furthest along in a phase III trial and the addressable global market for it is $1.5B.
Mino-Lok is an antibiotic lock solution used to treat catheter-related bloodstream infections (CRBSIs). CRBSIs, are serious especially in cancer patients receiving therapy through central venous catheters (CVCs) and in hemodialysis patients where venous access presents a challenge. In the pages below, I want to discuss what Mino-Lok is, how it could disrupt the Standard of Care (SOC), the status of the Mino-Lok Phase III clinical trial, and the market potential.
The current standard of care (SOC):
Mino-Lok is intended to salvage an infected CVC, reducing the need to remove and replace (R&R) the catheter. An alternative to the R&R procedure is a recognized unmet medical need. R&R is the SOC for CRBSI and occurs about 95% of the time after infection, according to CTXR. There are really no viable alternatives to removing and replacing the CVC once it becomes infected.
Studies show that R&R of CVCs has a 15% to 20% complication rate, including pneumothorax, misplacement, and arteria puncture. The cost to R&R a CVC is around $10K but can run from $30K to $75K in the cases with complications. Too high a number, 15% to 20% of the infected R&R procedures can end with morbidity.
In ICUs, doctors must have central line access continually. If a catheter becomes blocked, and can’t be cleared, it will be removed, and a new catheter will be placed somewhere in the body. Having a functioning CVC is vital for many ICU patients.
What is Mino-Lok?
Mino-Lok contains a proprietary combination of minocycline, edetate (disodium EDTA), and ethyl alcohol, all of which act synergistically to break down bacterial biofilms, eradicate the bacteria, and provide anti-clotting properties to maintain patency in CVCs, and salvage the infected catheter. These three compounds, combined to make Mino-Lok are already individually approved by the FDA.
Bacterial colonies can be surrounded in a biofilm that makes them resistant to antibiotics. Mino-Lok penetrates the biofilm, eradicates bacteria, and salvages infected, indwelling vascular catheters while providing anti-clotting properties. The Mino-Lok product is used in two-hour locking cycles, allowing the CVC to be used for its intended purposes for the remaining 22 hours each day. The product is infused into the CVC, held for 2 hours inside the catheter (locked), and then withdrawn. This occurs for 5-7 days until the catheter is clear.
There are currently no FDA-approved therapies to salvage infected CVCs. CTXR has the worldwide rights to Mino-Lok.
Aside from treating infected CVCs there is another potential application for Mino-Lok in its nascent stage. In an in vitro study, the Mino-Lok solution was found to be effective in eradicating Staphylococcus aureus, a notorious pathogen, and a leading cause of hospital-acquired infections.
The Mino-Lok Phase III clinical trial:
The Phase III clinical trial started in February 2018. That was some time ago but as you can imagine, the Mino-Lok clinical trial was slowed by the pandemic. The study is a randomized, open label, assess-blind study to determine the efficacy of Mino-Lok. 144 patients diagnosed with CRBSI are being randomized 1:1 into 1 of 2 treatment arms. The primary endpoint is Time to a catheter failure. The secondary outcome measures are, Proportion of subjects with overall success in the modified intent to treat (MITT), and clinically evaluable (CE) populations, Time to catheter failure in the MITT and CE Populations, Microbiological eradication, Clinical Cure, All-cause mortality and safety and tolerability.
The control arm for this trial consists of an alternative antibiotic lock comprised of the available therapy at the test sites based on standard practices or recommendations from the Infectious Diseases Society of America guidelines. This means that CTXR is willing to place Mino-Lok in competition with any antibiotic chosen by the sites.
Following a data review of safety and efficacy in June 2021, the independent Data Monitoring Committee (DMC) for the Mino-Lok® Phase III Trial recommended proceeding with the trial as planned. The DMC did not identify any safety concerns and no modifications were recommended to the protocol-defined sample size or power to achieve the primary endpoint.
Citius has continued to proceed in conducting largest controlled clinical trial to salvage infected catheters with no modifications requested by the DMC and no safety concerns identified. The company has guided that the Mino-Lok Phase 3 trial completion is anticipated by end of 2022.
The Market for Mino-Lok:
While there is nothing novel about the three individual components in Mino-Lok, the Company did secure a formulation patent for in 2018, which grants them protection until 2036. Mino-Lok also received QIDP. This potentially qualifies Mino-Lok for additional FDA incentives in the approval and marketing pathway, including Fast Track designation and Priority Review for development and a five-year extension of market exclusivity.
This means that CTXR may get another 5 years of market exclusivity beyond its 2036 patent expiration date. With the clinical trials nearing their finish and looking like Mino-Lok may get FDA approval, they will be the only FDA-approved treatment for infected CVC for all CRBSI for several years to come.
The market potential for Mino-Lok is estimated at $750 million per year in the U.S. and is projected to reach $1.84 billion globally in 2028. Of the approximately 7 million CVCs used annually in the US, up to 500,000 become infected and lead to CRBSIs. Mino-Lok has the potential to change the standard of care for the management of these serious infections.
Other CTXR blockbuster pipeline products in clinical trials:
I/ONTAK (E7777), a novel IL-2R immunotherapy for an initial indication in cutaneous T-cell lymphoma (CTCL), which has completed enrollment in its Pivotal Phase 3 trial. Citius anticipates filing a biologics license application (BLA) for I/ONTAK® with the U.S. Food and Drug Administration (FDA) in the second half of 2022. Topline data from the Phase 3 study of cancer immunotherapy I/ONTAK are consistent with the previously approved formulation of denileukin diftitox (ONTAK), and there are no new safety signals.
CITI-002 (Halo-Lido), a topical formulation of halobetasol, a corticosteroid, and lidocaine to provide anti-inflammatory and anesthetic symptomatic relief to hemorrhoids victims. It is currently in a Phase 2b trial initiated in April 2022 with last patient enrollment anticipated by the end of 2022.
CTXR products in pre-clinical development:
CITI-101 (Mino-Wrap), is a liquefying gel-based wrap containing minocycline and rifampin designed to provide inflammatory tissue protection and prevent infection and biofilm formation in tissue expanders and breast implants post-mastectomy.
Through its subsidiary, NoveCite, Inc., Citius is developing a novel proprietary mesenchymal stem cell treatment derived from induced pluripotent stem cells (iPSCs) for acute respiratory conditions (ARDS).
We will be back with more on CTXR soon,
The Traders News Group |