Apricus Biosciences, Inc. (NASDAQ: APRI) is a biopharmaceutical company advancing innovative medicines in urology and rheumatology. Apricus has two product candidates currently in development, Vitaros and RayVa.
On August 31st, APRI announced that the U.S. – FDA has acknowledged receipt of its recently resubmitted NDA for Vitaros™ (alprostadil, DDAIP.HCl) and considers it a complete, class 2 response to Apricus’ 2008 action letter.
The PDUFA (Prescription Drug User Fee Act) goal date for completion of the FDA’s review of the Vitaros NDA is set for February 17, 2018, which is the standard six-month review period for NDA re submissions.
Vitaros is a novel, on-demand topical cream for the treatment of erectile dysfunction (“ED”) and a new potential entrant into the U.S. ED treatment market. Vitaros has been approved in Canada, Mexico, and certain countries in Europe, Latin America and the Middle East, and the product is being commercialized by Ferring International Center S.A. and its licensees throughout Europe and in the Middle East.
Apricus in-licensed the U.S. development and commercialization rights for Vitaros from Allergan pursuant to a license agreement entered between the parties in September 2015. The U.S. Vitaros asset was previously purchased by Warner Chilcott, now a subsidiary of Allergan, from Apricus back in February 2009.
Pursuant to the terms of the license agreement, upon FDA approval of the NDA for Vitaros, Allergan may elect to exercise a one-time opt-in right to assume all future marketing and selling activities in the United States. If Allergan exercises its opt-in right, Apricus may receive up to a total of $25 million in upfront and potential launch milestone payments, plus a double-digit royalty on net sales of Vitaros. If Allergan elects not to exercise its opt-in right, Apricus may commercialize Vitaros and in return will pay Allergan a double-digit royalty on net sales of Vitaros.
Vitaros is a novel product with significant market opportunity. If approved, it is likely to address a significant unmet need in the erectile dysfunction market. Globally, ED is large market ($6.7B worldwide in 2016), out of which $3.5B ED market alone in the U.S with 20 million men estimated to be suffering from ED. As per Company estimate & assuming timely commercialization in 2018, the total Projected U.S. Net Sales would be around $150M in 2020.
The company’s business risk profile is on a rapid growth trajectory and is well positioned for a planned momentum. Furthermore, the company improved its financial outlook through a combination of fundraising and expense reduction, resulting in a balance sheet that is expected to fund current operating plan through the third quarter of 2018.
Importantly, APRI completed re-submission of Vitaros NDA with an anticipated FDA approval decision in the first quarter of 2018. For the remainder of 2017, the company focus is on working with the FDA regarding the Vitaros NDA, maintaining a productive dialogue with Allergan regarding the commercial potential for Vitaros in the United States, securing a development partner for RayVa and continuing to diligently manage its corporate resources.
Furthermore, The Company announced its earnings reports on August 2nd, 2017. Net loss for the quarter ended June 30, 2017 improved to $1.5 million, or loss per share of $0.13, compared to a net loss of $3.3 million, or loss per share of $0.54, for the second quarter of 2016. Net loss during the second quarter of 2017 was primarily due to expenses related to the preparation of resubmission of the Vitaros NDA and other general and administrative expenses.
The company’s stock has unsurprisingly found strength in the recent past and with the recent developments, analysts have revised their outlook on the stock. The stock currently has an average rating of “Buy” and a consensus price target of $1.72. Considering present valuation, the company is at a favorable risk reward position.
About the Company: Apricus Biosciences, Inc. (APRI) is a biopharmaceutical company advancing innovative medicines in urology and rheumatology. Apricus has two product candidates currently in development.
Key products:
Vitaros is a product candidate in the United States for the treatment of erectile dysfunction, which is in-licensed from Warner Chilcott Company, Inc., now a subsidiary of Allergan plc (Allergan).
RayVa is other product candidate in Phase 2 development for the treatment of the circulatory disorder Raynaud’s phenomenon, secondary to scleroderma, for which Apricus owns worldwide rights.
Key Highlights of Vitaros:
Novel Treatment for Erectile Dysfunction
- Development Stage in the U.S. Approved in parts of Europe, Canada, Latin America, and the Middle East.
- Only topically delivered treatment for erectile dysfunction in development
- Available in a single use 330 mcg dispenser
- Refrigeration required (2C – 8C)
Compelling Efficacy and Safety Profile
- Studied in over 3,300 patients
- Rapid onset (generally 5-30 minutes)
- Studied in diabetics, hypertensives, patients with cardiac issues or on nitrates/alpha blockers, prostatectomy patients and PDE-5 (e.g. Viagra®) failures
Strong IP Estate:
- Own or license issued patents that will expire from 2017 through 2032
Key Highlights of Rayva:
- Potential first-in-class topical cream treatment for Raynaud’s phenomenon Secondary to Scleroderma.
- Raynaud’s Phenomenon is an episodic vasoconstriction of the distal extremities affecting an estimated 3-5% of the U.S. population1,2
- Secondary Raynaud’s Phenomenon, affecting approximately many in the U.S. is driven by an underlying condition such as scleroderma which affects approximately 100,000.
- Increased incidence in women (approx. 80% of scleroderma patients), Triggers include cold, stress and vibration.
- Symptoms include pain, tingling, numbness, and coldness.
- Affected areas show at least two color changes: White (pallor), Blue (cyanosis), and Red (hyperemia), Brittle and ridged nails.
Potential value driver over the near to medium term:
2nd Quarter 2017 Financial Results:
Net loss for the quarter ended June 30, 2017 was $1.5 million, compared to a net loss of $3.3 million, or loss per share of $0.54, for the second quarter of 2016. Net loss during the second quarter of 2017 was primarily due to expenses related to the preparation of resubmission of the Vitaros NDA and other general and administrative expenses.
Net income for the six months ended June 30, 2017 was $6.6 million, or income per share of $0.69, compared to a net loss of $5.8 million, or loss per share of $1.00, for the second quarter of 2016. Net income during the six months ended June 30, 2017 was primarily due to the $12.1 million gain recorded for the sale of ex-U.S. Vitaros rights and assets to Ferring.
As of June 30, 2017, the Company’s cash totaled $7.8 million, compared to $2.1 million as of December 31, 2016.
Key risk factors and potential stock drivers:
Successful completion of the FDA’s review of the Vitaros NDA would lead the future direction for APRI. Any adversities related to these upcoming milestones might adversely impact the overall investor sentiments.
Also, significant product concentration continues to impinge the business risk profile of APRI. The company’s prospects largely depend on the positive outcome of clinical and commercial success of Vitaros. This dependence on a single product exposes it to a high degree of product & business concentration risks.
APRI is still at a pre-commercialization stage and has not yet generated meaningful revenue and will likely operate at a loss as it grows its market position and seeks ways to monetize it.
Therefore, any time or cost overrun in its ongoing R&D activities and its impact on the business & financial profile will remain a key business sensitivity factor. Moreover, meaningful commercialization of Vitaros is not likely to happen before 2nd quarter of 2018.
Stock Chart:
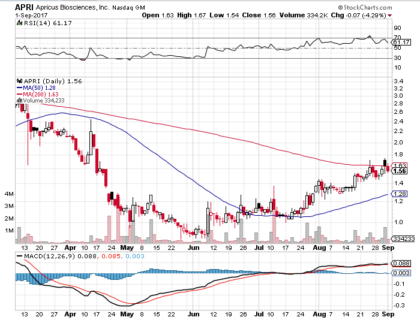
On Friday, September 1st, 2017, APRI closed at $1.56 (down by 4.29%) on an above average volume of 488,014.00 million shares exchanging hands. Market capitalization is $19.94 million. The current RSI is 61.17
In the past 52 weeks, shares of APRI have traded as low as $0.86 and as high as $4.94
At $1.56, shares of APRI are trading above its 50-day moving average (MA) at $1.28 and below its 200-day MA at $1.63
The present support and resistance levels for the stock are at $1.46 & $1.64 respectively.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP – SMS/text alert service for free, simply text the word “Traders” to the phone number “25827” from your cell phone***
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

