Tonix Pharmaceuticals Holding Corp. (TNXP) is developing innovative pharmaceutical products to address public health challenges. TNX-102 SL is in Phase 3 development and has been granted Breakthrough Therapy designation by the FDA for the treatment of post-traumatic stress disorder (PTSD). The company is headquartered in New York.
On March 29th, 2017, company announced that they would provide a corporate update and an overview of Tonix’s post-traumatic stress disorder (PTSD) clinical program at The MicroCap Conference on April 4, 2017 in New York, NY.
TNX-102 SL was recently granted break through therapy designation by the U.S. Food and Drug Administration (FDA) for the treatment of PTSD. This designation enables fast track development and review of a drug, which has potential for substantial improvement over available therapy.
As per management, Tonix is now in a strong position for value growth with Phase 3 development in a major medical indication i.e. PTSD including military-related PTSD. Moreover, Phase 3 HONOR study in military-PTSD is expected to initiate in 1Q 2017 & the interim analysis of the HONOR study is expected in the first half of 2018 and topline results are expected in the second half of 2018. Therefore, it is not too far from commercial operations.
To meet its incremental capital requirement, the company also announced a public offering of 1,800,000 shares of its common stock at a public offering price of $4.45 per share. The management intends to use the proceeds (around $8,010,000) from this offering to support the continued development of TNX-102 SL and other related activities.
Tonix’s business risk profile derives substantial strength through the series of positive developments in the recent past, which has been steadily encouraging for the company. Collectively, these developments suggest reasonably strong outlook for the company over the near to medium term.
However, notwithstanding these positive biases, it should also be noted that Tonix’s historical clinical trials have not been very successful/encouraging. Additionally, the company has raised significant equity in the past, causing dilution to its shareholders. Therefore, Tonix is substantially dependent on success of its present flagship product i.e. TNX-102 SL, and is exposed to the risk that it may not be able to commercialize the product.
Description & manufacturing set-up:
TNXP is developing innovative pharmaceutical products to address public health challenges. TNX-102 SL is in Phase 3 development and has been granted Breakthrough Therapy designation by the FDA for the treatment of posttraumatic stress disorder (PTSD).
Other development efforts include TNX-601 (tianeptine oxalate), a clinical candidate at Pre-IND (Investigational New Drug) application stage, designed for daytime use for the treatment of PTSD, and TNX-801, a potential smallpox-preventing vaccine based on a live synthetic version of horsepox virus.
Product Pipeline:
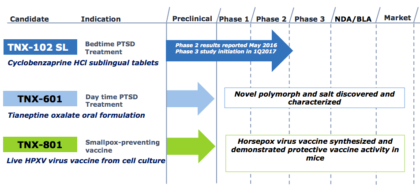
Source: Company presentation
Recent announcements:
- Announcements about TNX-102 SL: Tonix, will provide a corporate update and an overview of its posttraumatic stress disorder (PTSD) clinical program at The MicroCap Conference on April 4, 2017 in New York, NY. TNX-102 SL was recently granted Breakthrough Therapy designation by the U.S. Food and Drug Administration (FDA) for the treatment of PTSD.
In March 2017, Tonix dosed the first patient for the “HONOR” study, a 12-week placebo-controlled Phase 3 clinical study evaluating TNX-102 SL 5.6 mg, in military-related PTSD. The interim analysis of the HONOR study is expected in the first half of 2018 and topline results are expected in the second half of 2018.
TNX-102 SL Milestones – recent and upcoming:

- Announcements about public offering: Tonix has also announced a public offering of 1,800,000 shares of its common stock at a public offering price of $4.45 per share. The gross proceeds to Tonix from this offering are expected to be $8,010,000.
Tonix intends to use the net proceeds from this offering to support the continued development of TNX-102 SL for the treatment of PTSD, including the HONOR study in military-related PTSD, to further develop other pipeline programs, for working capital and other general corporate purposes, and possibly acquisitions of other companies, products or technologies, though no such acquisitions are currently contemplated.
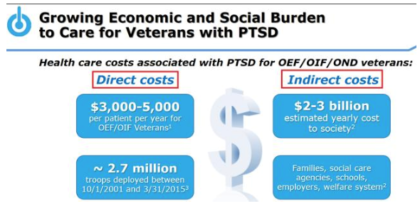
About PTSD condition: PTSD is a serious condition characterized by chronic disability, inadequate treatment options especially for military-related PTSD, and an overall high utilization of healthcare services that contributes to significant economic burdens. The Protectic™ protective eutectic and Angstro-Technology™ formulation are essential elements of the proprietary TNX-102 SL composition for which a Notice of Allowance has been issued by the U.S. Patent and Trademark Office.
Important target population & market size: U.S. veterans need a medicine that works for this serious condition. The market size of PTSD is estimated to be about $3 billion. CBO data shows that four years of PTSD treatment would cost roughly around $10,000.
Potential risk factors & key stock Influences over the near to medium term:
Exposed to project risk along with significant dependence on TNX-102 SL: The Company is still under pre- commercialization stage and is not likely to generate meaningful revenue until successful commercialization of their products occurs. Therefore, it is exposed to the risk associated with pre-commercialization process. In fact, in the past, Tonix had to freeze development of its fibromyalgia drug failed in late stage trial. After this setback, Tonix shifted its resources towards developing TNX-102 SL.
Business, financial condition and results of operations of Tonix, may be materially adversely affected by any delays in, or termination of, clinical trials or a determination by the FDA that the results of trials are inadequate to justify regulatory approval.
Negative profitability & subdued liquidity: As with any pre-development stage company, Tonix has experienced net losses and negative cash flows from operations since inception and expects these conditions to continue for the foreseeable future. Therefore, to fund operations, it needs to raise money through capital markets and/or private financing.
If additional financing is not available in a timely manner, Tonix may be required to delay, reduce the scope of or eliminate its research and development programs, reduce its commercialization efforts or obtain funds through arrangements with collaborative partners or others that may require it to relinquish rights to certain product candidates.
Furthermore, if it issues additional equity or debt securities, shareholders may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of common stock.
Earnings Review:
Tonix had no revenues or cost of goods sold during the three months ended September 30, 2016 and 2015.
Profitability:
The net loss for three months ended September 30, 2016 was $7.6 million, compared to a net loss of $13.3 million for three months ended September 30, 2015.
Research and development expenses for three months ended September 30, 2016 were $5.5 million, a decrease of $4.8 million, or 47%, from $10.3 million for the three months ended September 30, 2015. This decrease was primarily due to the winding down of the development work related to TNX-102 SL, including formulation development, manufacturing, human safety and efficacy studies.
Liquidity & capital resource:
As of September 30, 2016, Tonix had working capital of $26.6 million, comprised primarily of cash, cash equivalents and marketable securities of $26.7 million and prepaid expenses and other of $2.5 million, which was offset by $0.9 million of accounts payable and $1.7 million of accrued expenses.
Management expects to incur losses from operations for the near to medium term & future capital requirements will depend on several factors, including the progress of research and development of product candidates, the timing and outcome of regulatory approvals etc.
The company will need to obtain additional capital to fund future research and development activities. Future financing may include the issuance of equity or debt securities, obtaining credit facilities, or other financing mechanisms.
Stock Performance
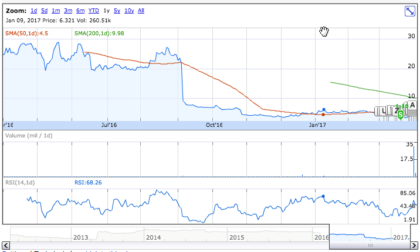
On Friday, March 31st, 17, Tonix shares increased by 0.65% to $4.67 on an average volume of 1.11M shares exchanging hands. Market capitalization is $27.66 million. The current RSI is 47.62
In the past 52 weeks, shares of Tonix have traded as low as $3.3 and as high as $37.70
At $4.47, shares of Tonix are trading below its 50-day moving average (MA) at $5.04 and 200-day MA at $9.9.
The present support and resistance levels for the stock are at $4.21 & $5.64 respectively.
About Traders News Source:
Big Opportunities in Small Cap’s
Traders News Source recent profiles and track record, 534% in verifiable potential gains for our members on 3 small cap alerts alone!
January 31st, 2017 (NASDAQ: HIMX) opened at $5.10/share and hit a high of $9.68/share March 24th, 2017 for gains of 89% within 60 days- http://finance.yahoo.com/news/himax-technologies-review-4q-2016-130000319.html
February 6th, 2017- (NASDAQ: SCON) opened at $1.12/share hit a high of $1.80/share within 10 days our member potential gains- 60% – http://finance.yahoo.com/news/superconductor-technologies-potential-revolutionize-smart-130000844.html
March 6th, 2017 (OTC: USRM) opened at .035/share and hit over .17/share within 25 days for gains of 385% for our members- http://finance.yahoo.com/news/traders-news-issues-comprehensive-report-130000743.html
These are numbers that make traders drool. Any trader in any market would fall all over themselves to see numbers like this. So, if you’ve been on the fence, perhaps it’s time to start doing some research and verify our numbers for yourself. We are constantly raising the bar and separate ourselves from the rest of the small-cap newsletters as the best in business.
We know with a large following comes a large responsibility as we have everyone from institutional investors to the beginner following our profiled securities in our newsletters. This is something we take very seriously always seeking small cap growth companies that have both near and long-term potential for our members.
***Get our small cap profiles, special situation and watch alerts in real time. We are now offering our VIP – SMS/text alert service for free, simply text the word “Traders” to the phone number “25827” from your cell phone.
Traders News Source Mission Statement
We strive to highlight the future potential as well as the inherent risk in each small cap company we cover while remaining neutral as a leading third-party equity research firm. Please read our privacy policy and full disclaimer below.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

