Trovagene, Inc. (NASDAQ: TROV) is a clinical-stage, oncology therapeutics company, using a precision medicine approach to develop drugs that target mitosis (cell division) to treat various types of cancer, including leukemias, lymphomas, and solid tumors. Trovagene has intellectual property and proprietary technology that enables the Company to analyze circulating tumor DNA (ctDNA) and clinically actionable markers to identify patients most likely to respond to specific cancer therapies.
The company is continuing to advance its clinical development of PCM-075 in Acute Myeloid Leukemia (AML) and Metastatic Castration-Resistant Prostate Cancer (mCRPC), achieving several key milestones in the recent past.
PCM-075 expanding pipeline and current stage:
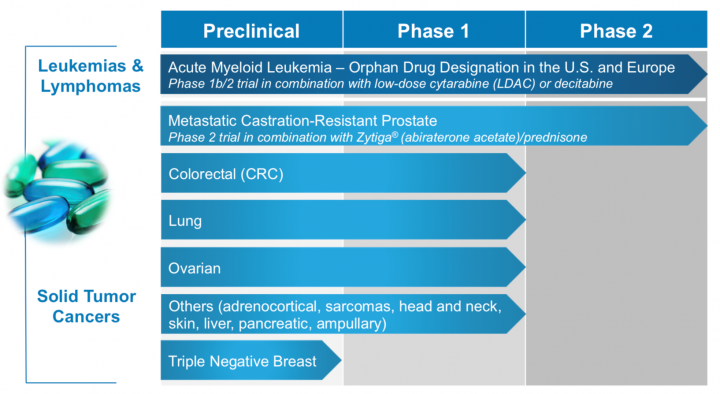
Key & unique differentiating factor:
► Precision Cancer Medicine, predictive biomarker approach
► Leveraging a proven cancer target, PLK1
► Onvansertib – first-in-class, 3rd generation, oral PLK1 inhibitor
► Synergy strategy – Onvansertib in combination with approved drugs
Recent announcements:
- Exclusive License Agreement with MIT for Combination Therapy of Anti-Androgens and Polo-like Kinase Inhibitors in Prostate Cancer: On Oct. 3, 2018, the company announced that it has entered into an exclusive patent license agreement with the Massachusetts Institute of Technology (MIT). Under the agreement, Trovagene has exclusive rights to develop combination therapies that include anti-androgen or androgen antagonist and a Polo-like Kinase (PLK) inhibitor for the treatment of cancer. The exclusive license agreement is part of the Company’s strategy to explore the efficacy of Onvansertib, its first-in-class, 3rd generation, highly-selective, oral PLK1 inhibitor, in combination with anti-androgen drugs in cancers including prostate, breast, pancreatic, lung and gastrointestinal.
- Completion of Dosing Cohort of Patients Treated with Onvansertib in Combination with Decitabine in Ongoing Phase 1b/2 AML Trial: On Sep 27th, the company announced completion of the second dosing cohort of Onvansertib, its first-in-class, 3rd generation, highly-selective oral Polo-like Kinase 1 (PLK1) Inhibitor, in combination with standard-of-care decitabine, in its Phase 1b/2 clinical trial in patients with Acute Myeloid Leukemia (AML). All three patients in the cohort successfully completed treatment with Onvansertib at 18mg/m2, administered orally, once daily, on days 1-5 of the treatment cycle, in combination with decitabine and the combination was well tolerated.
- Predictive Clinical Biomarker Approach to Identify Acute Myeloid Leukemia (AML) Patients Most Likely to Respond to Onvansertib: On September 5th, the company announced that it had developed a method for predicting response to treatment by measuring the ability of Onvansertib, a 3rd generation, oral and highly-selective Polo-like Kinase 1 (PLK1) inhibitor, to inhibit PLK1 in patients with Acute Myeloid Leukemia (AML). Trovagene has filed a U.S. patent application with the USPTO to protect its method for evaluating the responsiveness of cancer to a Polo-like Kinase 1 (PLK1) inhibitor by determining the ability of the PLK1 inhibitor to inhibit phosphorylation of a unique target of PLK1 in cells of the cancer.
______________________________________________________________________
Our members have booked up to 800% with our recent 2018 NASDAQ and NYSE small cap alerts. We will be initiating coverage on another exciting small cap security mid-week this week (10/14/18). View our recent picks, track record and sign up for our real time mobile/text alerts here – https://tradersnewssource.com/traders-news-source-new-members/
______________________________________________________________________
Looking ahead to 2019, the company is well poised for rapid growth. Trovagene is Integrating Technology in Tumor Genomics with the Development of Precision Cancer Therapeutics and the Analyst tracking the stock believes that the company’ deep understanding of tumor genomics may allow for effective targeting of appropriate cancer patients, which, in turn, may improve patient care and outcomes.
TROV has significant experience and expertise with biomarkers and technology in cancer, including AML. The company is the patent holder of NPM1 for diagnosis and monitoring of patient response. NPM1-mutated AML is a genetic marker in leukemia and accounts for approximately one-third of all AML patients.
Per www.marketbeat.com, Their average twelve-month price target is $27.50, suggesting that the stock has a possible upside of 3,060.92%. The high price target for TROV is $48.00, and the low-price target for TROV is $7.00. Considering all this, the company is in an extremely favorable risk-reward position, and value investors should consider exposure in this sector as the backdrop remains favorable.
Below are the excerpts of recent analyst rating on the script:
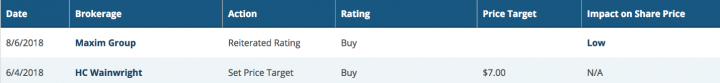
Source: marketbeat.com
Description & about the Company: Trovagene is a clinical-stage, precision medicine oncology therapeutics company. Trovagene’s lead drug candidate, PCM-075, is a Polo-like Kinase 1 (PLK1) selective adenosine triphosphate (ATP) competitive inhibitor.
PCM-075 was developed to have high selectivity to PLK1, to be administered orally, and to have a relatively short drug half-life of approximately 24 hours compared to other PLK inhibitors. PCM-075 has completed a safety study in patients with advanced metastatic solid tumors with a phase 1b/2 clinical trial in patients with AML underway.
PCM-075 has shown preclinical antitumor activity as a single agent and in synergy combinations with more than ten different chemotherapeutics and targeted therapies, such as Zytiga® (abiraterone acetate), Beleodaq® (belinostat), Quizartinib (AC220), a development stage FLT3 inhibitor, and Velcade® (bortezomib) in acute myeloid leukemia (AML), metastatic castration-resistant prostate cancer (mCRPC) and other liquid and solid tumor cancers.
2018 Milestones – Significant value-creation throughout 2018
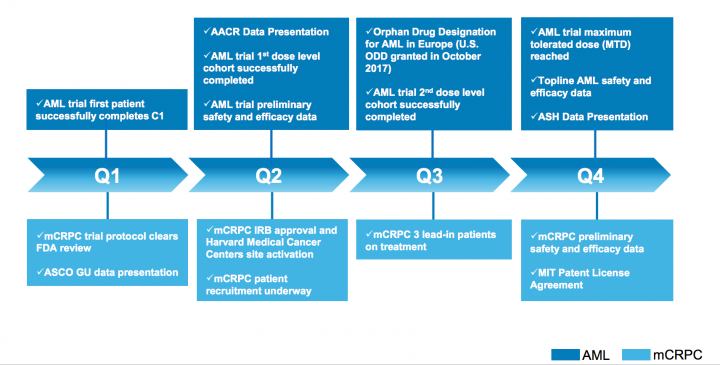
Other recent announcements:
- Announced Preliminary Clinical Data from First Dosing Cohort Demonstrating Durable Treatment Effect of PCM-075 in Combination with Cytarabine or Decitabine in Patients with Relapsed or Refractory AML
- Announced the Start of Recruitment and Enrollment for Phase 2 Clinical Trial of PCM-075 in Combination with Zytiga® in Patients with mCRPC
- Announced Completion of First Dosing Cohort of Patients Treated with PCM-075 in Combination with Decitabine in Ongoing Phase 1b/2 AML trial
- Announced Completion of First Dosing Cohort of Patients in Ongoing Phase 1b/2 AML trial of PCM-075 in Acute Myeloid Leukemia
- Announced Presentation of Data at AACR Meeting 2018 on Pharmacodynamic and Tumor Biomarkers During Treatment with PCM-075 and Low-Dose Cytarabine
- Announced Presentation of data at AACR Meeting 2018 Showing Synergy of PCM-075 in Combination with FLT3 Inhibitors in Acute Myeloid Leukemia (AML
Quarterly Financial Results (in, thousands):
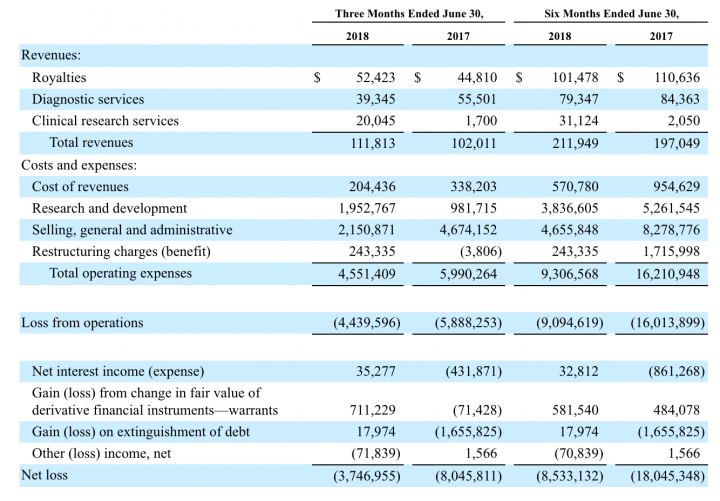
- Revenue – TROV’ total revenues were $111,813 and $102,011 for the three months ended June 30, 2018, and 2017, The increase in royalty income related primarily to higher receipts of payments in excess of minimum royalties in comparison to the same period of the prior year. Revenue from diagnostic services is recognized when payment is received for the test results. The amount of payments received was lower in 2018 as compared to the same period in the prior year.
- Profitability – Trovagene reported a net loss of $3.7 million and a net loss of $6.5 million attributable to common shareholders, or $0.88 per diluted share in the second quarter of 2018, as compared to a net loss of $8.0 million and a net loss of $8.1 million attributable to common shareholders, or $3.12 per diluted share for the same quarter of 2017.
- Liquidity and financial flexibility – As of June 30, 2018, Trovagene had approximately $18.5 million of cash and cash equivalents. Trovagene received gross proceeds of approximately $18.0 million from the sale of 9,140,000 shares of its common stock, 20,700,000 shares of warrants, and 8,600 shares of Series B Convertible Preferred Stock through an underwritten public offering that closed on June 12, 2018.
Key risk factors and potential stock drivers:
- Successful completion of the upcoming milestones would lead future direction for the company. Any adversities might adversely impact the overall investor sentiments.
- TROV has a history of operating losses. Therefore, any time or cost overrun in its ongoing R&D activities and its impact on business & financial profile will remain a key business sensitivity factor.
- Notwithstanding the expected improvement in the business and financial risk profile of the company, TROV is still a loss-making entity. Therefore, the company’s ability to achieve successful commercialization will continue to remain a long-term stock sensitivity factor.
Stock Performance
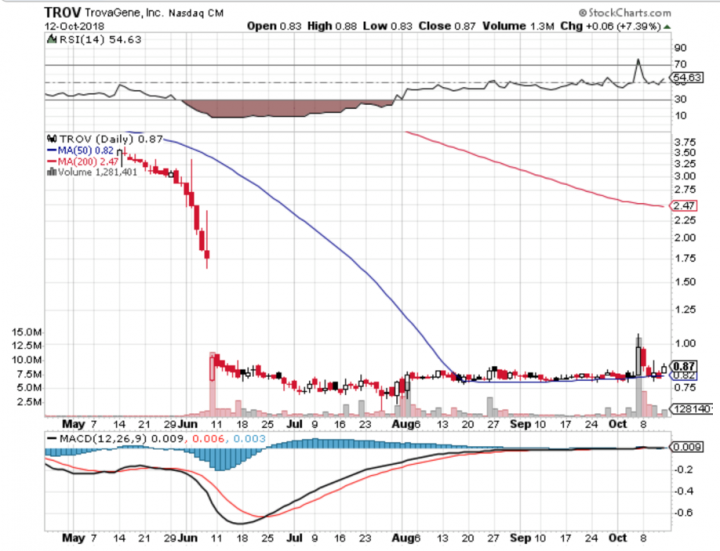
Comments:
- On Friday, Oct 12th, 2018, TROV closed at $0.8656, with a volume of 1.3 million shares exchanging hands. Market capitalization is $15.311 million. The current RSI is 54.63
- In the past 52 weeks, shares of TROV have traded as low as $0.65 and as high as $12.00
- At $0.8656, shares of TROV are trading above its 50-day moving average (MA) at $0.82 and below its 200-day moving average (MA) at $2.47
- The present support and resistance levels for the stock are at $0.81 & $0.91 respectively.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Vikas Agrawal, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

