Our february 22nd, 2017 initial coverage of Celsion (NASDAQ: CLSN) can be viewed here- https://tradersnewssource.com/celsion-corp-cancer-medications-insider-buying-and-analysis/
June 7th, 2017 Celsion Analyst Report
Company Overview
Celsion Corporation (NASDAQ: CLSN) is a development stage oncology company. Celsion’s portfolio of cancer treatments includes directed chemotherapies, DNA-medicated immunotherapy, and RNA-based therapies. The company’s lead product candidate is ThermoDox, a proprietary dosage of doxorubicin based on a heat-activated liposomal platform, currently in currently in a Phase III clinical trial for the treatment of primary liver cancer. The development pipeline also includes GEN-1, a DNA-based immunotherapy which is currently in a Phase I clinical trial for the treatment of ovarian cancer.
Products
As noted above, Celsion is conducting various clinical trials for its two leading product candidates, ThermoDox and GEN-1. The company’s product pipeline is detailed in the table below:

Source: Company Website
ThermoDox uses proprietary liposome technology to encapsulate doxorubicin, a commonly used cancer drug. The heat-sensitive liposome rapidly changes structure when heated to between 40 and 45 degrees Celsius, creating openings in the liposome that release doxorubicin directly into and around the targeted tumor. ThermoDox, has been shown to deliver 25 times more doxorubicin into tumors than intravenous transfusion alone, and 5 times more doxorubicin than standard liposomal formulations of the drug.
GEN-1 is the first product designed using Celsion’s TheraPlas technology platform. TheraPlas has shown to be an effective immunotherapy treatment for various types of tumors when utilized in combination with interleukin-12 (IL-12) plasmid, one of the most active cytokines for stimulating an immune response against cancer.
However, the pharmacokinetics of IL-12 requires that it be administered by frequent, large bolus injections, resulting in serious toxicities that limit its use. Using persistent and local secretion of IL-12 at therapeutic levels, GEN-1 is able to address these toxicity issues. GEN-1 has shown positive results for the treatment of ovarian, colorectal, and brain cancer.
A Phase I study (OVATION Study) of newly diagnosed ovarian cancer patients is currently underway to determine the maximum tolerated dose of GEN-1 as a neoadjuvant therapy to first-line standard of care, followed by surgery. The study is also evaluating dose-related biological response to IL-12.
Market Overview
Ovarian cancer is the most lethal gynecological malignancy with an overall five-year survival rate of 45 percent. This is primarily attributable to the lack of effective prevention and early detection strategies. There were approximately 22,000 new cases of ovarian cancer in the U.S. in 2014, with an estimated 14,000 deaths. Mortality rates have declined only slightly in the last 40 years due to the lack of effective testing and treatments. Most women are not diagnosed until the cancer reaches Stage III or IV, which is when the disease spreads outside the pelvis. Five-year survival rates for these cases range from 11 to 41 percent.
Recent Developments
On June 5, 2017, Celsion announced the latest clinical and translational data from its OVATION Study. At the American Society of Clinical Oncology annual meeting, Dr. Premal H. Thaker indicated that the study produced highly promising clinical findings, including a patient with a complete pathological response and a very high rate of R0 (complete remission).
Data from the first 14 patients who completed treatment in the OVATION Study demonstrated that GEN-1 plus standard chemotherapy produced positive results with no dose limiting toxicities, and promising dose dependent efficiency signals. Celsion also reported the following outcomes from the study:
- Of the 14 patients treated in the study, two demonstrated a complete response, 10 demonstrated a partial response, and two demonstrated a stable disease. The five patients in the high-dose cohort produced a 100 percent objective response rate, with one complete response and four partial responses;
- All 14 patients had successful resections of their tumors, with nine achieving R0 resections. All five patients in the high dose cohort had R0 resections;
- One patient demonstrated a pathological complete response, which is seen in less than seven percent of patients receiving neoadjuvant chemotherapy followed by surgery;
- All patients experienced a clinically significant decrease in their CA-125 protein levels as of their most recent study visit. CA-125 is present in greater concentrations in ovarian cancer cells than in other cells.
First Quarter Earnings Review
The company does not yet generate revenues from the sale of products. The company’s only revenue in the first quarter of 2017 was $125,000 of licensing revenue, which is connected to a $5 million technology transfer payment being recognized over 10 years. Celsion’s operating expenses, consisting of research and development and general and administrative expenses, decreased seven percent year-over-year to $4.9 million. Other expenses were roughly flat. Accordingly, the company’s 2017 first quarter net loss was $5.2 million, or $0.12 per share.
Net cash used in operations fell 35 percent year-over-year to $3.1 million. At March 31, 2017, the company listed cash and equivalents of $4.5 million and notes payable of $1.5 million. The company’s working capital deficit was $4.2 million.
Celsion’s most recent capital raise was a secondary public offering in February 2017, raising gross proceeds of $5 million.
Stock Influences
- Further developments regarding ThermoDox or GEN-1;
- Exercise of warrants and further equity offerings to raise cash;
- Licensing or other agreements to raise revenue; and
- M&A activity.
Risk Factors
- The company does not expect to generate revenues from products for the foreseeable future;
- The company will need to raise additional capital to fund its operations;
- The company needs to obtain approvals from the FDA and foreign regulatory bodies to market its products; and
- The company’s stock price remains volatile and subject to speculation.
Stock Performance
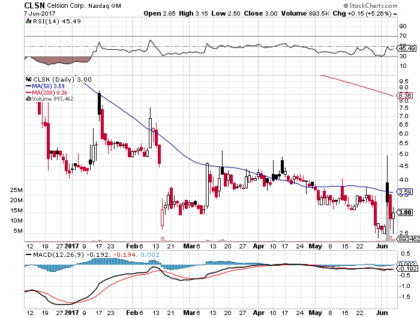
As of June 5, 2017, shares of Celsion closed at $3.30, gaining 24 percent on the day, yielding a market capitalization of approximately $14 million. Following the disclosure of the results from the OVATION Study, shares hit an intraday high of $4.96 before settling at $3.30. In the week prior to the announcement, shares traded at a one-year low of $2.40.
On May 30, 2017, Celsion completed a 14-for-1 reverse stock split. Over the same period, Celsion’s annualized daily volatility was 109 percent.
Trading volume has increased significantly in the past year. The average daily volume in May 2017 was 100,000, as compared to less than 5,000 in the same month last year.
Summary
Traders News Source is updating its report on Celsion issued February 22, 2017 after positive interim results from the OVATION Study and insider buying activity (https://tradersnewssource.com/wp-content/uploads/2017/02/CLSN-Analyst.pdf). While the stock has experienced some volatility, it has been mainly up since that time. Furthermore, the recently announced results from the OVATION Study caused the stock to jump more than 74 percent in intraday trading.
Celsion’s research appears promising, but to continue its research and commercialize its products, Celsion will need to raise additional financing.
Disclaimer
Traders News Source is a wholly owned subsidiary of Traders News Source LLC, herein referred to as TNS LLC.
Traders News Source has not been compensated for this report by anyone and the opinions if any are that of the author Ivan Neilson, CFA. Author’s Disclosure: I have no positions in any stocks mentioned, and no plans to initiate any positions within the next 72 hours. I, wrote this article myself, and it expresses my own opinions. I have no business relationship with any company whose stock is mentioned in the article.
This web site, published by TNS LLC, and is an investment newsletter that is built on the premise of assisting individual investors in learning about investing. Our goal as publishers of financial information is to provide research and analysis of investments to our subscribers. TNS LLC does not give buy or sell recommendations. We do purchase distribution rights from analyst, financial writers and bloggers for a fee that may be licensed to issue price targets and recommendations. Furthermore, we encourage you to speak to a licensed professional prior to making an investment in any type of publicly traded security.
We do sell advertising to other companies including brokerage firms, web sites, publicly traded issuers, investor relations firms, and investment publications, among others. TNS LLC makes no warranty as to the policies of these organizations, and in no way endorses their offers, services, or the content of their advertisements.
When an advertiser is a publicly traded company or a third party acting on behalf of a public company, we fully disclose all compensation in the email advertisement. Such disclosure is included in a disclosure statement in each of the advertisements sent via email.
17B Disclosure
Our reports/releases are a commercial advertisement and are for general information purposes ONLY. We are engaged in the business of marketing and advertising companies for monetary compensation. Never invest in any stock featured on our site or emails unless you can afford to lose your entire investment. The disclaimer is to be read and fully understood before using our services, joining our site or our email/blog list as well as any social networking platforms we may use.
PLEASE NOTE WELL: TNS LLC and its employees are not a Registered Investment Advisor, Broker Dealer or a member of any association for other research providers in any jurisdiction whatsoever.
Release of Liability: Through use of this website viewing or using you agree to hold TNS LLC, its operator’s owners and employees harmless and to completely release them from any and all liability due to any and all loss (monetary or otherwise), damage (monetary or otherwise), or injury (monetary or otherwise) that you may incur. The information contained herein is based on sources which we believe to be reliable but is not guaranteed by us as being accurate and does not purport to be a complete statement or summary of the available data. TNS LLC encourages readers and investors to supplement the information in these reports with independent research and other professional advice. All information on featured companies is provided by the companies profiled, or is available from public sources and TNS LLC makes no representations, warranties or guarantees as to the accuracy or completeness of the disclosure by the profiled companies. None of the materials or advertisements herein constitute offers or solicitations to purchase or sell securities of the companies profiled herein and any decision to invest in any such company or other financial decisions should not be made based upon the information provide herein. Instead TNS LLC strongly urges you conduct a complete and independent investigation of the respective companies and consideration of all pertinent risks. Readers are advised to review SEC periodic reports: Forms 10-Q, 10K, Form 8-K, insider reports, Forms 3, 4, 5 Schedule 13D.
TNS LLC is compliant with the Can Spam Act of 2003. TNS LLC does not offer such advice or analysis, and TNS LLC further urges you to consult your own independent tax, business, financial and investment advisors. Investing in micro-cap and growth securities is highly speculative and carries an extremely high degree of risk. It is possible that an investor’s investment may be lost or impaired due to the speculative nature of the companies profiled.
The Private Securities Litigation Reform Act of 1995 provides investors a ‘safe harbor’ in regard to forward-looking statements. Any statements that express or involve discussions with respect to predictions, expectations, beliefs, plans, projections, objectives, goals, assumptions or future events or performance are not statements of historical fact may be “forward looking statements”. Forward looking statements are based on expectations, estimates, and projections at the time the statements are made that involve a number of risks and uncertainties which could cause actual results or events to differ materially from those presently anticipated. Forward looking statements in this action may be identified through use of words such as “projects”, “foresee”, “expects”, “will”, “anticipates”, “estimates”, “believes”, “understands”, or that by statements indicating certain actions & quote; “may”, “could”, or “might” occur.
Understand there is no guarantee past performance will be indicative of future results. In preparing this publication, TNS LLC has relied upon information supplied by its customers, publicly available information and press releases which it believes to be reliable; however, such reliability cannot be guaranteed. Investors should not rely on the information contained in this website. Rather, investors should use the information contained in this website as a starting point for doing additional independent research on the featured companies. The advertisements in this website are believed to be reliable, however, TNS LLC and its owners, affiliates, subsidiaries, officers, directors, representatives and agents disclaim any liability as to the completeness or accuracy of the information contained in any advertisement and for any omissions of materials facts from such advertisement. TNS LLC is not responsible for any claims made by the companies advertised herein, nor is TNS LLC responsible for any other promotional firm, its program or its structure.

